Semiconducting nanocrystals called colloidal quantum dots (CQDs) are ideal for applications such as large-panel displays and photovoltaic cells thanks to their high efficiency and colour purity. Their main drawback is their toxicity, since they have traditionally been made from cadmium or other heavy metals, such as lead. Researchers at the Los Alamos National Laboratory in the US have now engineered cadmium-free QD solar cells that reach efficiencies on par with those of their environmentally-unfriendly counterparts. The key to the new devices’ high performance is their tolerance to defects, they say.
CQDs can be synthesized in solution, which means that films of these nanocrystals can be deposited quickly and easily on a range of flexible or rigid substrates – just like paint or ink. Such semiconducting nanocrystals are ideal for making highly-efficient inorganic solar cells that emit light via a process known as radiative recombination. Here, an electron in the valency energy band in the QD absorbs a photon and moves to the conduction band, leaving behind an electron vacancy, or hole. The excited electron and hole then recombine, releasing a photon.
The advantage of using CQDs as photovoltaic materials in solar cells is that they absorb light over a broad spectrum of solar radiation wavelengths. This is because the band gap of a CQD can be tuned over a large energy range by simply changing the size of the nanocrystals. Such a size-tuneable property has allowed the efficiencies of these QDs to rapidly approach those of traditional thin-film photovoltaics, such as PbS, CdTe and Pb-halide perovskite QDs.
Free from toxic elements
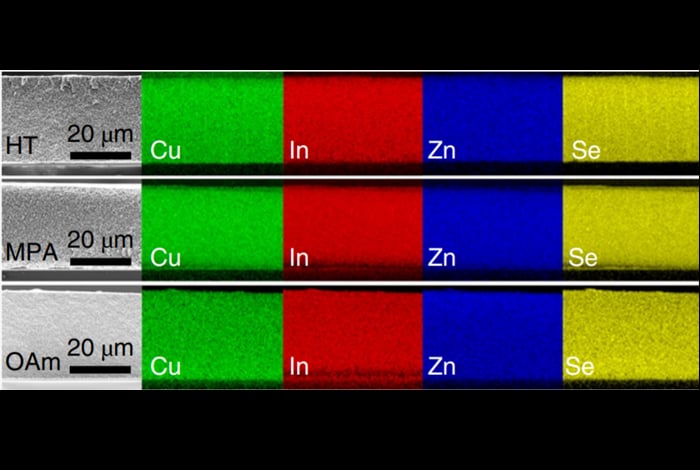
A team of researchers led by Victor Klimov have now developed high-efficiency QD solar cells that are free from any toxic elements. They made their new devices by reacting copper, indium and selenium and then adding zinc to the mix to produce zinc-doped QDs. They then incorporated these QDs into voids of a highly porous titanium dioxide (TiO2) film, which plays the part of a charge-collecting electrode. The electrode was immersed in a Na2S electrolyte.
When the QDs in the device absorb incoming photons from sunlight, tightly bound electrons in the valence band get excited into a high-mobility conduction band. These electrons subsequently migrate to the TiO2 electrode, generating a current in the process.
The researchers say they were “pleasantly surprised” by the results of photovoltaic and spectroscopic measurements on the new devices. Because their QDs have a very complex composition – four elements are combined in the same nanosized particle – they are prone to (intragap) defects, Klimov explains. These defects act as traps in which electrons get stuck, which means that electrons and holes have time to recombine instead of being whisked apart to produce useful current.

Quantum dot LEDs go cadmium-free
“However, despite these imperfections, the ZCISe QDs showed near perfect performance in the new solar cells,” says Klimov. “Per each 100 absorbed photons, we detected 85 photogenerated electrons, implying that the photon-to-electron conversion efficiency was 85%.”
Defect-mediated photoconversion
According to the Los Alamos team, who report their work in Nature Energy, the defects in their material actually help the photoconversion process along, rather than impeding it. In particular, the material’s high photovoltaic efficiency stems from a peculiar mechanism involving two types of intragap defects, they explain. The first, identified as shallow surface-located electron traps, enhance electron transfer between the QDs and TiO2 electrode. The second, identified as Cu1+ hole-trapping defects, help transfer holes between the QDs and the electrolyte.
The traditional assumption that surface defects are always detrimental thus does not seem to hold true for ZCISe QDs, they say. Indeed, the structures may even be protecting electrons and holes from unwanted recombination. This implies that surface traps are electronically coupled to the TiO2 electrode and, importantly, that the energy of these traps is high enough for electrons to transfer efficiently into the TiO2 conduction band.
The high photoconversion efficiencies, combined with the remarkable defect tolerance and toxic-element-free composition of these QDs makes them promising materials for a commercially viable solar-cell technology, they conclude.