A new software quantification tool has been developed by researchers in the US for analyzing white-matter abnormalities in very preterm babies. This magnetic resonance imaging (MRI) biomarker can predict motor development risks, including cerebral palsy, in a much more objective way than existing diagnostic tools.
Today, most children born extremely prematurely (earlier than 30 weeks of gestation) survive. However, nearly 70% of these very preterm infants show abnormalities in the white matter of their brains. Furthermore, half of very prematurely born children develop minor motor abnormalities, and up to 10% develop cerebral palsy (CP).
CP is a group of permanent neurological disorders that affect movement, posture, and balance. It is the most common physical disability in children. Even though the last three decades have brought improved survival and care for extremely preterm babies, they are about 50 times more likely to develop CP than full-term babies. Improved care for these children has not translated into fewer disabilities, since most children with CP are diagnosed at 1–2 years of age and conventional neuroimaging is subjective and not sensitive on its own to accurately predict CP.
Early intervention
Yet, identifying biomarkers of CP at an early stage of life could significantly increase the quality of life for both affected patients and their families. Early intervention by physical and occupational therapy could take advantage of early neuroplasticity and mitigate symptoms such as tremors, stiff muscles, or difficulty performing precise movements.
To address these issues, a group of researchers led by Nehal Parikh, a neonatologist at the Perinatal Institute, Cincinnati Children’s Hospital Medical Center, has been developing better diagnostic tools using advanced imaging risk factors to improve outcome prediction. They have developed an algorithm that can analyze structural MRI scans of preterm infants to predict their risk of motor development abnormalities based on spotting and quantifying brain defects.
Fully automated detection
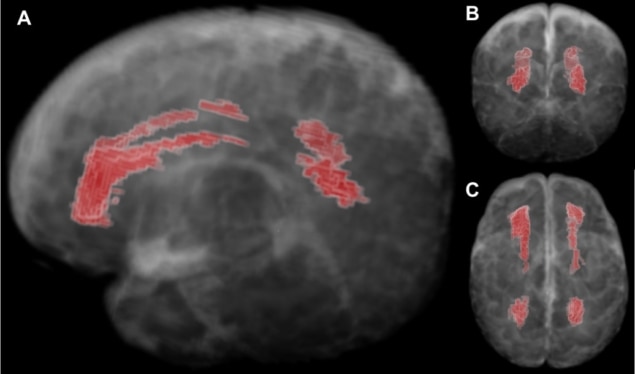
Parikh’s team targeted diffuse white matter abnormality (DWMA), the most common finding in premature infants. It appears on a brain MRI scan as areas with increased signal intensity. Early evidence suggests these abnormalities are associated with inflammation-initiating illnesses and may represent areas of increased fluid in the brain’s wiring.
Current tools to assess DWMA rely on visual analysis of the MRI data. “Manual tracing of DWMA regions within the brain is highly subjective and produces poor reliability and reproducibility,” explains Parikh. “It is clear that we need a better tool for diagnostics. I started working with computer scientists to come up with an algorithm that can segment these lesions.”
In the study, the team enrolled very prematurely born infants and performed brain structural MRI scans at the term-equivalent age. These images were fed into an algorithm created using a probabilistic brain atlas constructed specifically for a very preterm infant brain.
“Since it was not possible to create a gold standard manual brain atlas for DWMA, Lili He, a computer scientist in my lab, decided to use a synthetic one to train the algorithm. I drew out the respective brain regions manually,” explains Parikh. The resulting algorithm could detect signal intensity and location of normal white matter, gray matter, and cerebrospinal fluid and isolate DWMA regions based on their different signal intensity and spatial information with an accuracy greater than 95% when compared with the ground truth.
Towards clinical translation
Critics might say that the algorithm represents a computer simulation trained on a synthetic brain model that is not relevant to a real patient. However, it can already predict clinical outcomes relevant to patients and their families. Indeed, the team correlated their results with cognitive and language scores of their cohort at the age of two; and motor scores at three years of age. “The results correlated very nicely,” says Parikh. “It showed that DWMA volume was predictive of standardized developmental scores up to three years of age, independent of other conventional MRI and other known predictors.”
The team is now working on implementing a deep learning approach to improve the DMWA region identification. In addition, Parikh is planning to work with leading MRI vendors to implement the algorithm into their consoles to allow for the earliest diagnostics possible.
Virtual imaging trials aim to optimize COVID-19 screening
“Ideally, when the MRI scan is complete, our algorithm would do its job within seconds and provide a value for diffuse white matter abnormality,” he says. “If this value was abnormal, the child would be referred for aggressive early intervention therapies and/or research trials of novel interventions.”
Manon Benders, a neonatologist at the Wilhelmina Children’s hospital, UMC Utrecht, foresees a big future for this research as a decision-support tool. “It is very important since obtaining the most accurate prognosis as early as possible is essential in order to inform the child’s family adequately.”
“Moreover, beginning the intervention therapy even before the onset of clinical symptoms of cerebral palsy or adverse motor outcomes is also crucial, particularly given that the brain’s plasticity is highest in the first few months after birth,” she adds.
These research is described in Scientific Reports.
