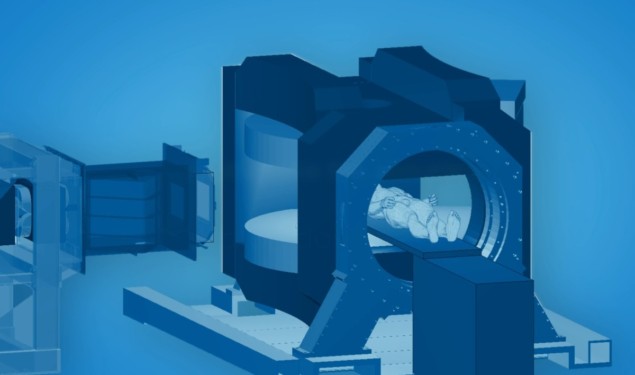
Proton therapy is an advanced cancer treatment technique that delivers highly targeted dose to the tumour while sparing surrounding normal tissue, enabled by the finite range of the proton beam. This precision targeting, however, is compromised by tumour motion or anatomical changes throughout a course of treatment. The absence of fast imaging tools to localize moving targets during dose delivery is a fundamental barrier to exploiting the full potential of proton therapy.
Real-time imaging during treatment delivery could visualize the tumour and synchronize the proton beam to its motion. MRI, which has recently been integrated into conventional photon-based radiotherapy systems, could provide high-resolution, high-contrast soft-tissue imaging, without depositing any additional ionizing dose into the patient. But operating an MRI scanner in conjunction with a proton beam is a major technological challenge that, for a long time, many considered to be impossible.
Aswin Hoffmann from the HZDR Institute of Radiooncology – OncoRay in Dresden thought otherwise. Hoffmann and his colleagues have been working for several years to integrate MRI with proton therapy. Now, the team is planning to build the world’s first whole-body prototype proton therapy system that can track moving tumours with MRI, in real time, during dose delivery from an actively scanned proton pencil beam.
The major challenge when integrating MRI into a proton therapy system is that MRI scanners need precisely defined magnetic fields to create geometrically accurate images, while proton therapy systems use electromagnetic fields to generate, transport and deliver the proton beam. Interference between these fields could distort the MR image and impact the delivered proton dose distribution. Hoffmann and his team showed that it is technically possible to combine both systems, and that these interference effects can be anticipated and thus compensated for. They also recently demonstrated that the proton beam range can be visualized with online MRI.
The prototype system will incorporate a 0.5 T rotating open MRI scanner produced by ASG Superconductors, which uses a helium-free, superconducting magnesium diboride magnet. The MRI scanner has been adapted to meet the requirements of real-time MRI-guided therapy by MagnetTx Oncology Solutions, a spin-off of the Alberta Health Services LINAC-MR group that developed the Aurora RT MR-guided radiotherapy system. Engineers at MagnetTx are also developing a gantry to rotate the scanner, as well as image processing methods to automatically track the tumour in real time.
In the summer of 2022, the team plans to incorporate the MRI system into a clinical-grade, actively scanned proton beamline at OncoRay.
The design of the new proton therapy system is based on the state-of-the-art Aurora RT. “As the Aurora RT has been optimized for image-guided radiation treatment, our prototype system will leverage its unique features to provide real-time image guidance for treatment with high-precision proton beams,” Hoffmann tells Physics World. “Our vision is to not only use it clinically for high-precision cancer treatments, but also for other pathologies that can be targeted non-invasively with highest precision comparable to surgical procedures.”
The MRI scanner will enable real-time, high-contrast imaging of organs in the thorax, abdomen and pelvis. Another advantage is that the scanner can be rotated around the patient relative to the proton beam. This will enable the team to study dosimetric and biological beam effects of MRI magnetic fields both perpendicular and parallel to the proton beam.

MR-guided proton therapy: a status update
“MR-integrated proton therapy will have the capability to capture anatomical changes during therapy and allow for treatment adaptations to increase the targeting precision and reduce normal-tissue side effects,” explains Hoffmann. “The main benefit is expected for the treatment of tumours that show motion during irradiation, such as liver, pancreas, oesophagus, kidney, adrenal and cervical cancers.”
“Thanks to the collaboration with international industrial partners, my team and I are a big step closer to our goal of bringing significant innovation to the field, especially to real-time image-guided proton therapy,” he adds.
