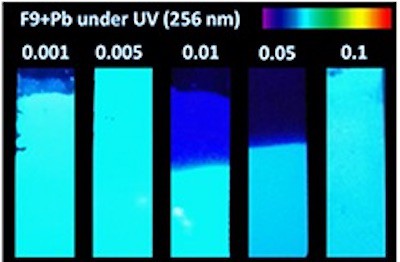
Researchers in Mexico have used zeolites – porous aluminosilicate minerals widely used in water purification – to detect toxic lead in water for the first time. The team’s new one-step technique, which can rapidly detect lead concentrations as low as 1.248 parts per billion, could be extended to other heavy metals.
Lead causes serious health problems for humans and poses a hazard for the environment. Together with mercury, arsenic, chromium and cadmium, it is one of the five most common heavy-metal contaminants in water. Although many highly sensitive spectrometry and spectroscopy techniques exist for detecting it, most require sophisticated instruments and complicated sample preparation methods.
Zeolites are hollow three-dimensional structures made from aluminium, oxygen, silicon and alkali or alkaline-earth metals such as sodium, potassium and magnesium. They contain large, regularly arranged micron-sized pores that can absorb water and remove heavy metal ions from it thanks to a mechanism known as ion exchange, which occurs when one type of ion in a solution is reversibly exchanged with another type of ion that has the same electrical charge. Zeolites’ pores also make ideal “cages” in which to stably isolate sub-nanoscale clusters of metals.
Thanks to the electrons that easily jump from one discrete energy level within the metal clusters to another, these “metal-exchanged” zeolites make efficient light emitters. As such, they already have applications as fast, highly sensitive optical sensors for environmental monitoring. However, zeolites had not been used to detect toxic metal ions until Eduardo Coutino-Gonzalez and colleagues at the Centro de Investigaciónes en Óptica in Guanajuato set out to exploit their ion-exchange properties for this purpose.
Synthesizing fluorescent clusters
The researchers made their metal-exchanged zeolites using an aqueous lead acetate solution containing different concentrations (0.001, 0.005, 0.01, 0.05 and 0.1 M) of lead (Pb). They then thermally activated the structures by heating them to 450°C. This process produced fluorescent lead clusters within the zeolites via reduction and migration of the lead ions.
In a series of photoluminescence measurements (detailed in Journal of Physics: Photonics), Coutino-Gonzalez and colleagues found that the intensity of the light emitted from the materials depends strongly on the concentration of lead in the sample being analysed. Remarkably, they observed maximum emission intensities at low lead concentrations. This, they say, shows that lead-exchanged zeolites make efficient and highly-sensitive fluorometric sensors for Pb2+ ions.
Novel nanosheet allows for efficient molecular sieving
Simpler, cheaper, faster
“Our method provides a simpler, cheaper and faster approach to detect lead ions in water at very low concentrations compared to conventional analytical techniques used for quantitative detection of lead ions, Coutino-Gonzalez tells Physics World. Unlike other recently-developed fluorescent chemosensors, he adds, the team’s method requires only a single step. “In our proposal, zeolites are used as adsorbents to confine lead ions and, at the same time, as scaffolds to convert lead ions into highly luminescent lead clusters,” he explains.
The researchers now plan to explore the limits of their method by testing for even lower lead concentrations and comparing their results with those obtained using established techniques. “We will also be trying to detect different types of heavy metal ions in water as well as investigating other zeolite frameworks, such as natural zeolites,” Coutino-Gonzalez says. “These would further reduce the cost of sensors based on these structures.”