Two independent teams have taken inspiration from nature to develop better ways of producing hydrogen with solar cells. The first team, from the University of Michigan in the US, achieved a record-breaking solar-energy-to-hydrogen efficiency of more than 9% using a solar panel containing an indium gallium nitride (InGaN) catalyst. The second team, from the EPFL in Switzerland and Toyota Motors Europe, created a new type of transparent, porous gas diffusion electrode that harvests water from the air and turns it into hydrogen when exposed to sunlight. Both technologies could, in principle, supply hydrogen for fuel cells and industrial processes in a “green” way, without the need for fossil-fuel precursors.
In photocatalytic solar water splitting, scientists use the energy from sunlight to split water into its component elements: oxygen and hydrogen. This process mimics a crucial step in natural photosynthesis and could be a clean and renewable way to produce energy. The problem is that the efficiency of the solar-to-hydrogen (STH) process is very low, making it uneconomical compared with fossil-fuel-based methods of generating the large quantities of hydrogen needed for various industrial processes.
9.2% efficiency
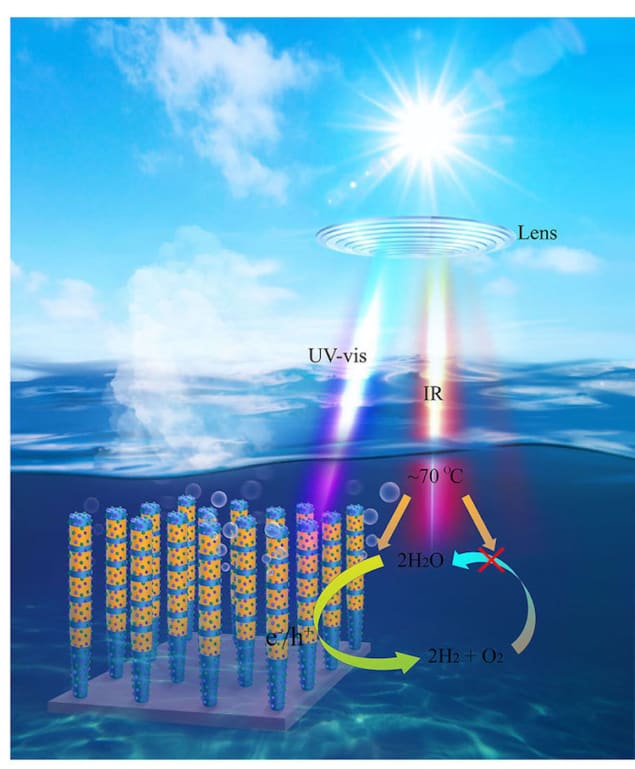
At Michigan, Zetian Mi and colleagues used the ultraviolet-to-visible part of the Sun’s spectrum to photoexcite the semiconductor InGaN, causing it to produce electrons and “holes” (regions of positive charge) that can split water into hydrogen and oxygen. The researchers then used the infrared portion of sunlight to heat the reaction system to around 70 °C. This heating helped to prevent the hydrogen and oxygen from recombining to form water, which is the main “backreaction” in water splitting and a major limiting factor in STH efficiency.
The result of this strategy was a STH efficiency of around 7% for tap water and sea water and 6.2% in a large-scale prototype photocatalytic water-splitting system located outdoors. Indoors, the system achieved an efficiency of 9.2% – 10 times greater than previous solar-powered water-splitting experiments of the same type.
The device, which the team describe in Nature, is also stable at high temperatures and at light intensities equivalent to 160 Suns. “In principle, this technology can supply hydrogen for fuel cell stations and any industrial processes that require hydrogen,” Mi tells Physics World. “A unique advantage of this approach is the distributed generation of hydrogen, compared to conventional centralized steam methane reforming processes, thereby significantly reducing the cost associated with hydrogen transportation.”
Like an artificial leaf
The gas diffusion electrodes made by Marina Caretti and colleagues in the EPFL team, meanwhile, are based on quartz (silicon dioxide) fibres processed into felt wafers that are then fused together at temperatures of 1350 °C. The team coated the resulting transparent porous substrates with a transparent thin film of a photoactive material, fluorine-doped tin oxide, in an atmospheric chemical vapour deposition process for 10 min at 600 °C with monobutyltin trichloride and trifluoroacetic acid.
The structure thus produced has a porosity of 90%, giving it the maximum amount of contact with water vapour in the air and a good conductivity of 20 ± 3 Ω sq−1. It is also transparent, allowing light to pass through the coated semiconductor.
When exposed to sunlight, this device behaves like an artificial leaf, harvesting water from the air and using sunlight to produce energy (in the form of hydrogen gas, in this case). The energy from the solar radiation is stored in hydrogen bonds, similar to the way that plant leaves store energy in the chemical bonds of sugars and starches produced during photosynthesis.
Technology could harvest humidity from the air
The EPFL researchers, who detail their work in Advanced Materials, acknowledge that the solar-to-hydrogen conversion efficiency of their photoelectrochemical (PEC) device is quite low. However, maximizing this efficiency was not a goal of their study, and team member Kevin Sivula points out that its maximum theoretical efficiency is around 12%. This, he says, indicates “promise for improvement”.
“The system concept will also eliminate the need for a highly acidic electrolyte, traditionally employed in PEC devices,” he tells Physics World.

Light-activated catalysts make nearly perfect water-splitters
The EPFL team’s prototype was only stable for about an hour under one Sun’s worth of illumination, which Sivula says will “need to be improved” to make the device practical. One possible application for it might be in PEC cells, which use incident light to stimulate a photosensitive material such as a semiconductor that is immersed in liquid solution. The goal in this case is to drive chemical reactions, but the process has some drawbacks – one being that it is complicated to make large-area PEC devices that use such a solution. The new work shows that PEC technology can be adapted to harvest humidity from the air instead.
Optimization in progress
The EPFL researchers are now seeking to optimize their system by studying different fibre and pore sizes and different semiconductor materials. They are pursuing their work in the context of the EU Project Sun-to-X, which is dedicated to advancing this technology.
The Michigan researchers, for their part, now plan to separate the pure hydrogen from mixed hydrogen and oxygen produced in their photocatalytic water splitting process using a membrane. “We will also develop a new photocatalytic approach to directly produce high-purity hydrogen from water splitting,” Mi says.
