
An unusual phase of ice that may be responsible for a spectacular atmospheric effect called a Scheiner’s halo has been observed in its pure form for the first time. Known as cubic ice, the phase develops when water freezes on low-temperature interfaces, and researchers in China have now confirmed its existence using transmission electron microscopy. The finding advances our understanding of ice and could be used to develop more accurate models of ice behaviour.
Ice is found on Earth in many forms, and the role it plays – for example, in cloud physics and climate change – depends strongly on its structure. The main type of naturally-occurring ice is hexagonal ice (Ih), so-called because the water molecules arrange themselves in a hexagonal lattice (this is the reason why snowflakes have six-fold symmetry). Under certain conditions, however, ice can form other structures. Indeed, 20 different forms of ice have been identified so far.
One of these 20 forms is cubic ice (Ic), which has a diamond-cubic structure with a repeating face-centred cubic array of tetrahedrally-coordinated water molecules. This type of ice is believed to play a role in the formation of Scheiner’s halos, which are extremely rare and always observed in the sky at an angle of 28° from the Sun or Moon. However, the existence of cubic ice has long been a subject of debate, as it proved difficult to detect pure-phase Ic unambiguously in experiments.
Monitoring ice formation in real time
In the new work, researchers led by Lifen Wang and Xuedong Bai of the Beijing National Laboratory for Condensed Matter Physics and the Songshan Lake Materials Laboratory in Dongguan used an imaging technique called in situ cryogenic transmission electron microscopy (TEM) to monitor the formation of ice crystallites in real time.
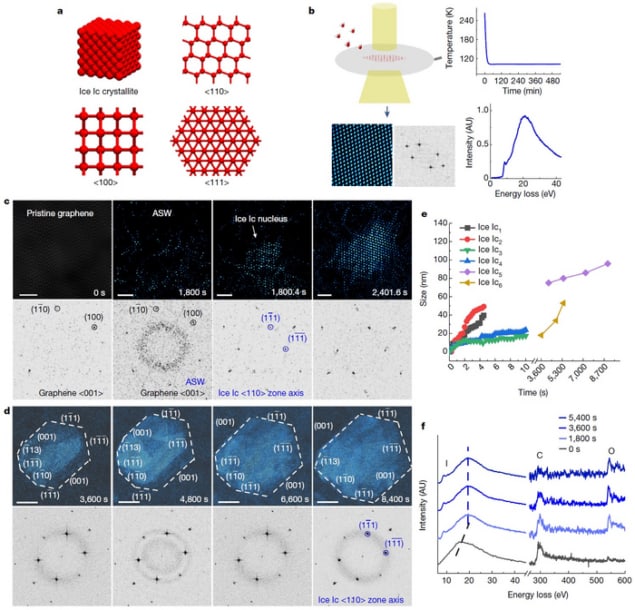
In their experiments, the ice formed on a freeze plate made from monolayer graphene at temperatures ranging from room temperature to liquid nitrogen temperature. As the researchers decreased the plate’s temperature, the residual water vapour in the vacuum chamber of the microscope gradually condensed – similar to the way water vapour in the air transforms directly into solid ice on cold days, forming a thin layer on surfaces such as windowpanes or windscreens.
The TEM images, which Wang, Bai and colleagues describe in Nature, reveal that depositing amorphous solid water onto the cold plate led to the formation of Ic nuclei, which have a single-crystalline lattice along the <110> zone axis. The researchers measured a lattice constant a in these crystals of 6.36 angstroms, which is similar to the diffraction value of 6.37451 angstroms for heavy-water ice Ic. The Ic nuclei grow to form a faceted crystallite in a few hours and the size of these crystallites is in the nanometre range. The observations imply that the resulting ice is pure-phase I, with a majority being single crystalline ice Ic together with a small amount of ice Ih.
Wang says that being able to track ice at molecular resolution in this way advances our understanding of ice and its properties. “Our study provides new insights into its formation behaviour, shedding fresh light on its structure, growth and defect dynamics,” she tells Physics World. “This knowledge could be used to develop more accurate models of ice behaviour, which could have important implications for fields such as climate and environmental science.”

Thin ice reveals its secrets
The insights gained from the study of ice on low-temperature interfaces could also be exploited to develop materials with enhanced ice-repellent properties for applications in aviation, transportation and other industries, she adds.
Looking to the future, the researchers say they now plan to study how different ice polymorphs compete with each other during crystallisation, and to explore the microscopic mechanisms that lead to the formation of the variety of ice crystals observed in nature.
