RaySearch Laboratories and its clinical customers are leveraging advances in machine learning to reimagine the radiotherapy workflow
Machine-learning technologies are unleashing a wave of data-driven innovation and transformation in radiation oncology, yielding step-function improvements in automation, workflow efficiency and consistency of treatment – both for individual clinics and across multicentre healthcare systems. Writ large, the end-game of data-driven oncology represents a compelling narrative – one that will be elaborated in detail for visitors to the booth of RaySearch Laboratories, the Stockholm-based oncology software company, at the annual congress of the European Society for Radiotherapy and Oncology (ESTRO) in Vienna, Austria, later this week.
“Clinical collaboration and model validation are essential for the successful deployment of machine learning in the planning, delivery and management of radiotherapy treatment programmes,” explains Fredrik Löfman, director of machine learning at RaySearch. What Löfman is alluding to, specifically, is the at-scale collection and aggregation of data for model development from RaySearch’s international user base, while partnering closely with clinical experts on tasks like data enrichment and data curation to ensure robust validation of machine-learning models for the vendor’s flagship RayStation treatment planning system (TPS).
Front-and-centre on the RayStation innovation roadmap are automated deep-learning segmentation (DLS) and deep-learning-enabled automation in treatment planning. “The priority is to work with medical physicists and radiation oncologists to improve, optimize and generalize the machine-learning models in RayStation over their life-cycle,” adds Löfman. “After all, it’s the clinics that provide the real-world evaluation and validation of machine learning measured in terms of treatment quality and patient outcomes.”
Streamlined segmentation
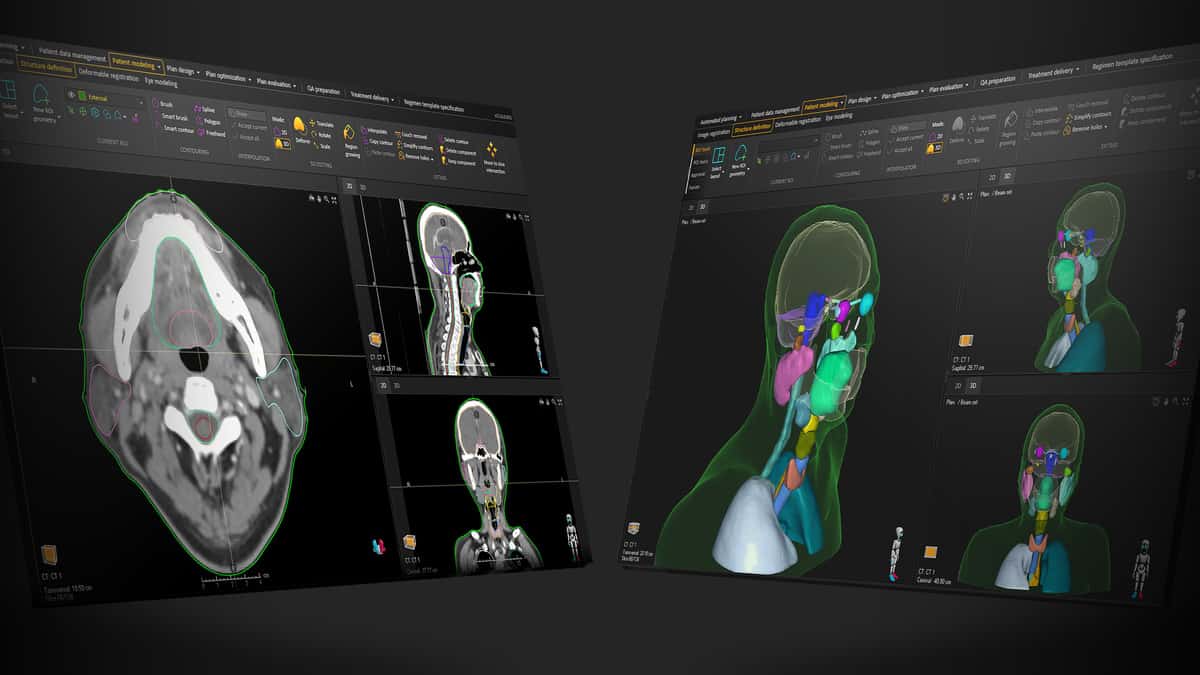
That process of clinical validation is already well under way – and accelerating. Consider the commercial roll-out and clinical trajectory of DLS, with a growing number of treatment centres fast-tracking the clinical adoption of RayStation’s catalogue of DLS models for automated segmentation of diverse disease indications spanning head-and-neck/brain, thorax and breast, abdomen and pelvis – in some cases, reducing the time spent on patient contouring by as much as 75% versus manual or semi-automatic methods.
Put simply, RayStation’s DLS functionality – trained and validated on large-scale patient data sets – automatically creates contours of critical structures in the tumour near-environment. Clinical teams are then able to review and fine-tune the segmentation in order to optimize tumour control and reduce radiation toxicity.

Last year, a case study in this regard saw the training, validation and clinical implementation of RayStation DLS models for radiotherapy of loco-regional breast cancer – a collaboration between RaySearch, St Olavs Hospital (Trondheim, Norway) and Ålesund Hospital (Ålesund, Norway). The joint team trained DLS models for 18 structures (including breast lymph nodes) on 170 left-sided breast-cancer cases; another 30 patient cases were used for validation. Based on the first two months of clinical experience, the treatment centres reduced total delineation time from roughly one hour to 15 minutes per patient, while the DLS models also out-performed manual segmentation methods in terms of the consistency and standardization of contouring.
“The DLS methodology, algorithms and ‘infrastructure’ are an integral part of RayStation,” explains Löfman. “As such, DLS is a natural extension of the TPS and modelling of patients, with patient data always remaining within RayStation and no need for users to export image data and import results.” What’s more, the DLS catalogue is growing with every model release and will ultimately cover all of the main disease sites for radiotherapy treatment. An enhanced prostate model will go live in June, for example, while models for head-and-neck lymph nodes are another development priority this year.
Planning horizon
Downstream from DLS in the RayStation workflow, Löfman and his cross-disciplinary team – 20 scientists and engineers split across planning, imaging and analytics subgroups – are also pressing ahead with the clinical roll-out of deep-learning-enabled automation in treatment planning. Here, RayStation’s machine-learning models are used to predict and optimize 3D spatial dose, with in-built strategies to automatically generate a set of deliverable treatment plans across key modalities, including intensity-modulated radiotherapy (IMRT), volumetric modulated arc therapy (VMAT), helical tomotherapy and pencil-beam-scanning treatment systems.
Operationally, fast-track comparison of those candidate plans is followed by selection of the optimal plan for each patient in terms of tumour coverage, conformality and tissue-sparing. In this way, the radiation oncology team can quickly review the plans for each patient, pick the most suitable, and then fine-tune (automatically, semi-automatically or manually) if needed.
“We now have over a dozen centres using RayStation’s deep-learning-enabled treatment planning clinically on a regular basis – saving lots of time and effort in the process,” explains Löfman. “Working with our customers, we have proved that deep-learning technology delivers robust, high-quality plans automatically – for both photon and proton treatment systems and a range of disease sites spanning prostate, lung, breast, head-and-neck and rectum.”
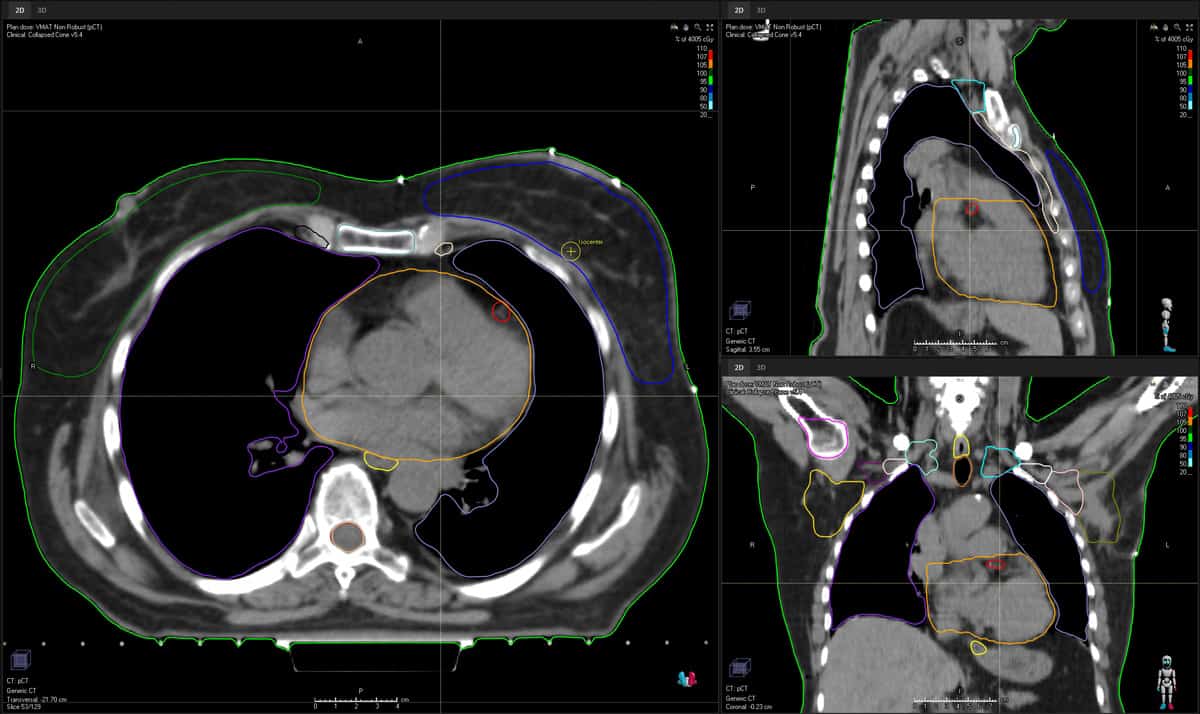
RaySearch, for its part, works closely with end-users to configure deep-learning planning models to local treatment protocols and clinical preferences, while deployment into the radiotherapy workflow is a multistep process designed to streamline the path to clinical translation. In the first instance, RaySearch engineers will validate the model prior to release (a mix of quantitative and qualitative assessment), with the model scope and limitations subsequently shared with the customers. After which the clinic will commission the model (evaluating its performance on local data) ahead of approval and live implementation in the radiotherapy treatment chain.
“It is essential to consider the full life-cycle of the clinically deployed deep-learning models,” notes Löfman. “Right now, for example, we are collaborating with key clinical partners to initiate a systematic programme of evaluation looking at model performance over time.”
Meanwhile, delegates attending the ESTRO exhibition will be able to see the latest RayStation innovations up close – with one eye-catching product demonstration highlighting the operational upside of integrating DLS and deep-learning-enabled planning within a unified TPS environment. Starting with the CT image of a prostate case, the demonstration will show how DLS can fast-track segmentation of all critical structures and the prostate to automatically generate the target volumes. The DLS output then feeds seamlessly into the VMAT plan set-up, using a deep-learning model to automatically generate a deliverable, high-quality plan. “This is a game-changer,” claims Löfman. “The DLS and treatment planning take somewhere between four to five minutes end-to-end with only a single user-click to initiate the process.”
Clinical intelligence
Notwithstanding the headline focus on machine learning, Löfman is also pushing the importance of “big data” as an enabler of clinical best practice in radiation oncology – and specifically the availability, accessibility and standardization of patient and workflow data to support optimized treatments and enhanced patient outcomes. At the heart of that collective conversation is RayIntelligence, the vendor’s cloud-based oncology analytics system, which combines consolidated data warehousing as well as structuring, transformation and dashboarding of the resulting centralized data repository for easier consumption and analysis.
“RayIntelligence is all about helping clinics to become more data-driven,” notes Löfman. “In other words: using data collected during the ‘patient journey’ to deliver personalized care that’s grounded in real-world evidence.” There is a gap, he argues, for this sort of data warehousing capability, such that users will be able to visualize and drill down into their patient and workflow data in near-real-time to facilitate benchmarking, outlier detection and continuous process improvement.
Long term, Löfman also sees opportunities for RayIntelligence to provide the infrastructure and tools needed for evaluation of machine-learning models on relevant patient cohorts. He concludes: “Innovation in machine learning requires large-scale data sets that researchers, clinics and industry can access in an unbiased and representative way. RayIntelligence provides the building blocks needed to centralize – and allow models to learn from – the vast amounts of data generated by multicentre clinical trials.”