
A team of US-based researchers has used an innovative head-mounted device to shrink a brain tumour – potentially paving the way for a powerful new non-invasive therapy for glioblastoma.
In recent studies, the team – which includes researchers based at the Peak Center for Brain and Pituitary Tumor Treatment and Research at Houston Methodist Neurological Institute – found that the oscillating magnetic field-generating device, dubbed an “oncomagnetic device”, was able to rapidly kill glioblastoma cells in culture and shrink human glioblastoma tumours implanted in mice, prolonging their survival. The device was also used to shrink an end-stage recurrent glioblastoma in a patient without access to any other approved treatment option. The researchers describe the results of this case study in Frontiers in Oncology.
As co-author Santosh Helekar from Houston Methodist Research Institute explains, the portable, wearable device consists of “strong permanent magnets rapidly spun by high-speed electric motors, whose rotation and timing are controlled by a programmable microcontroller operated by a rechargeable battery”. The magnet and motor assemblies are housed in vibration-, sound- and heat-insulated enclosures mounted on a helmet worn by the patient. A treatment-specific pattern of rotation frequencies and timings is then used to stimulate the brain to treat glioblastoma.
Glioblastoma is the most common cancer of the brain. Helekar observes that advances in its treatment have prolonged the median survival of patients newly diagnosed with the disease only slightly – from nine months four decades ago to “about 15 to 20 months today”.
Promisingly, this single-patient case report demonstrated that a month-long course of oncomagnetic treatment – during which the oscillating magnetic field was applied for two hours, up to three times a day on weekdays – reduced the volume of end-stage recurrent glioblastoma by more than 30%.
Helekar notes that the new technique is currently being used in both research and clinical settings under the auspices of an ongoing research project supported by the Translational Research Initiative of the Houston Methodist Research Institute.
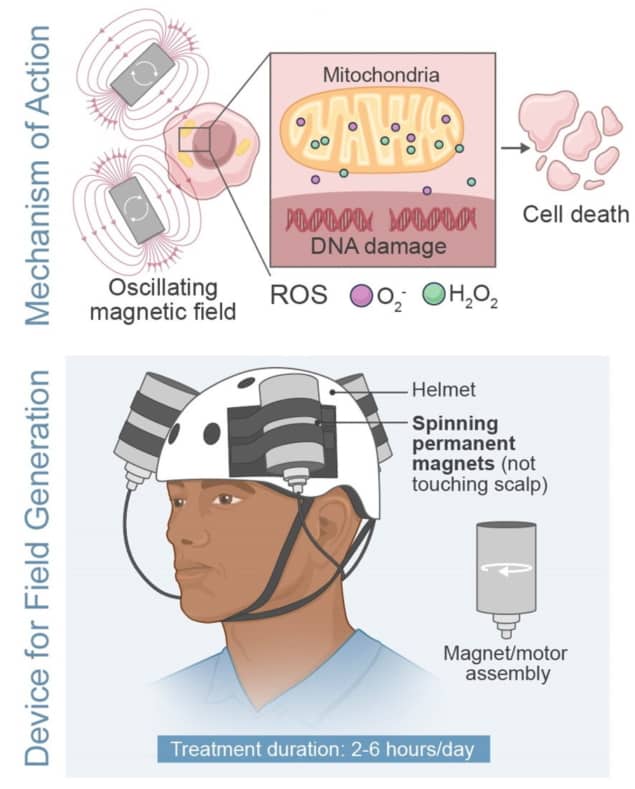
“Our recently published laboratory research findings show that the oncomagnetic device kills glioblastoma and other cancer cells in culture by increasing reactive oxygen species [ROS] in the mitochondria and cytoplasm of these cells, while sparing non-cancerous cells, such as neurons, astrocytes and bronchial epithelial cells,” Helekar explains.
“We hypothesize that the increase in reactive oxygen species is at least in part due to magnetically induced disruption of the electron flow in the mitochondrial electron transport chain,” he continues. “The rotating magnetic fields influence the spins of unpaired electrons exchanged by free radical intermediates in the chemical reactions taking part in the unmovable transmembrane protein complexes of the electron transport chain. Confirmation of some of the predictions of this hypothesis are going to be published shortly.”
Next steps
One key advantage of the new device is that, in contrast with existing treatments for glioblastoma, it does not have any known serious side effects. Moreover, it does not involve drug treatment nor require shaving of the head.
“The total duration of daily treatment on weekdays is only up to six hours,” Helekar says. “It is likely to be much less expensive because of the low cost and simplicity of the device. The device is very easy and convenient to use because it simply involves the wearing of a helmet up to three times a day.”

Multimodal spectroscopy detects brain tumours in vivo
Moving forward, the team is currently undertaking laboratory-based preclinical studies of the device to test its biophysical, cellular and molecular mechanisms of action on cells in culture, as well as its safety and efficacy in mouse models of glioblastoma.
“With David Baskin, Peak Center director and vice-chair of neurosurgery as the principal investigator, we are also continuing the FDA-approved compassionate use treatment of patients with end-stage recurrent glioblastoma, like that reported in the recently published case report,” says Helekar. “Our plans are to obtain regulatory approval for a pilot clinical trial to test the safety and efficacy of the device for the treatment of glioblastoma. Furthermore, we plan to collaborate with other institutions nationally and internationally to conduct similar trials in other cancers.”
