The fundamental building blocks of human tooth enamel contain characteristic impurities that could contribute to their toughness, but also make teeth more vulnerable to decay. US researchers led by Derk Joester at Northwestern University made the discovery using two atomic-scale imaging techniques, which revealed distinctive distributions of impurities within the crystal structure of enamel. Their findings could lead to new ways to improve the health of our enamel, and to repair the damage created by tooth decay.
Coating the entire crown of the human tooth, enamel is an extremely hard substance that is well adapted to withstanding the wear and mechanical forces associated with chewing. Yet in many people, the material is broken down through tooth decay – which is one of the most widespread chronic diseases. So far, techniques for repairing and synthesizing new enamel have had limited success, largely due to limitations in our understanding of the material’s deeply hierarchical structure.
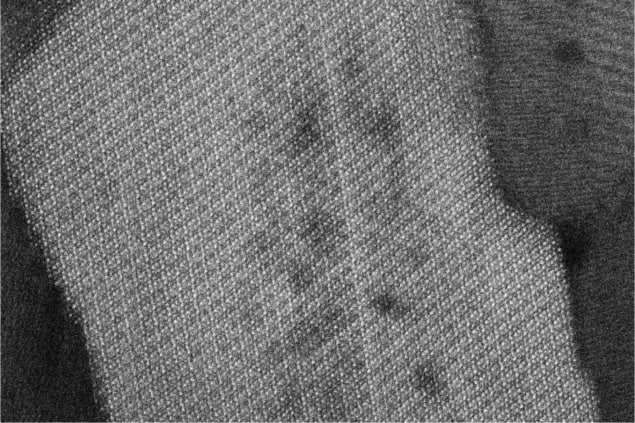
At the microscopic level, enamel is made up of rods composed of thousands of long, thin crystals with rectangular cross-sections – called crystallites. The rods are arranged in complex meshess. No more than 170 nm wide, the crystallite building blocks are primarily composed of the mineral hydroxyl-apatite. This has an orderly lattice containing calcium, phosphate, and hydroxyl ions. However, the crystallites are also known to possess cross-sectional variations in chemical composition on atomic scales.
Atom-probe tomography
To explore these variations in more detail, Joester’s team first carried out scanning transmission electron microscopy (STEM) on thin, ultra-cold crystallite cross-sections. The resulting images revealed that within the periodic lattice of hydroxyl-apatite, crystallites have central cores with slightly different compositions. These cores are sandwiched between two distinctive layers (see figure). Since these structures were damaged by STEM electron beams, Joester’s team also employed the technique of atom-probe tomography (APT), which can locate individual atoms at sub-nanometre resolutions. Within these images, they saw that crystallite cores contain high concentrations of sodium, fluoride, and carbonate ion impurities, and are flanked by two magnesium-rich layers.
Complex tooth development is explained by simple diffusion-limited growth
Through simulations, combined with experiments involving X-ray diffraction, Joester and colleagues revealed that these chemical gradients create stress patterns in enamel, which could be partly responsible for the materials’s high mechanical resilience. However, the impurities could also increase the solubility of the material in acidic conditions, making it more vulnerable to decay. The team’s findings could offer important new insights into the crystal growth that takes place during enamel formation, and how it could be controlled artificially. If achieved, such techniques could lead to new treatments for people suffering from tooth decay, and to new ways of maintaining healthy enamel.
The study is described in Nature.
