Radiation therapy is a complex procedure, with a series of equipment and dosimetry checks performed before every treatment to ensure its safety and accuracy. However, there’s still potential for errors to occur during the actual radiation delivery, such as changes in patient geometry, inaccuracies in beam delivery or mispositioning of brachytherapy sources.
In vivo dosimetry (IVD), which measures the dose to the patient during the treatment, could detect any such errors and help ensure the accurate delivery of radiotherapy. But its adoption in clinical practice has so far been low.
In November 2017, at the first ESTRO Physics Workshop, a task group was created to investigate this low uptake and stimulate the wider adoption of IVD. After three years of work, the task group has now published its recommendations for the future development and clinical use of IVD in the two most common forms of radiotherapy: external-beam photon radiotherapy (EBRT) and high dose rate (HDR) or pulsed dose rate (PDR) brachytherapy.
“Our task group believes there should be more and tighter checks on radiotherapy, in particular during the beam delivery to the patient,” explain Frank Verhaegen from Maastro Clinic and Kari Tanderup from Aarhus University Hospital, who led the EBRT and brachytherapy teams, respectively. “IVD is one of those techniques that all clinics could have, the equipment is available, but hardly anyone does it. We analysed the reasons for this and tried to come up with technical requirements and guidelines for equipment manufacturers and clinical users.”
Issues arising
The project, which integrated academics, clinicians and equipment vendors from Europe, North America and Australia, was coordinated by Gabriel Fonseca and Jacob Johansen (for brachytherapy), and Igor Olaciregui-Ruiz (for EBRT). The first challenge was to create a formal definition for IVD. After much debate, the group agreed that “IVD is a radiation measurement that is acquired while the patient is being treated, containing information related to the absorbed dose in the patient”. As such, an IVD system must be able to detect errors arising from equipment failure, dose calculation errors, anatomical changes, and patient (EBRT) or applicator (brachytherapy) positioning errors.
Writing in an editorial in phiRO, Verhaegen and Tanderup detail the key requirements identified for IVD. In addition to acting as a safety system to catch errors that could affect the patient, an IVD method should also provide tools for treatment adaptation and record the true dose received by the patient. Ideally, an IVD system should record signals in real time without perturbing the dose to the patient.
But with such potential to improve radiotherapy, why is IVD is so under-utilized? The task group suggests that many clinics do not perform IVD because they consider the clinical benefit to be too low, or because workflows are too complex and resource-heavy. Manufacturers, meanwhile, are unwilling to invest due to limited demand from clinics and a lack of regulations.
“It’s a bit of a chicken-and-egg problem,” says Verhaegen. “There are quite a few products that one can buy, but they all only do part of the work. And because there is little guidance on how to use it, people just don’t use it.”
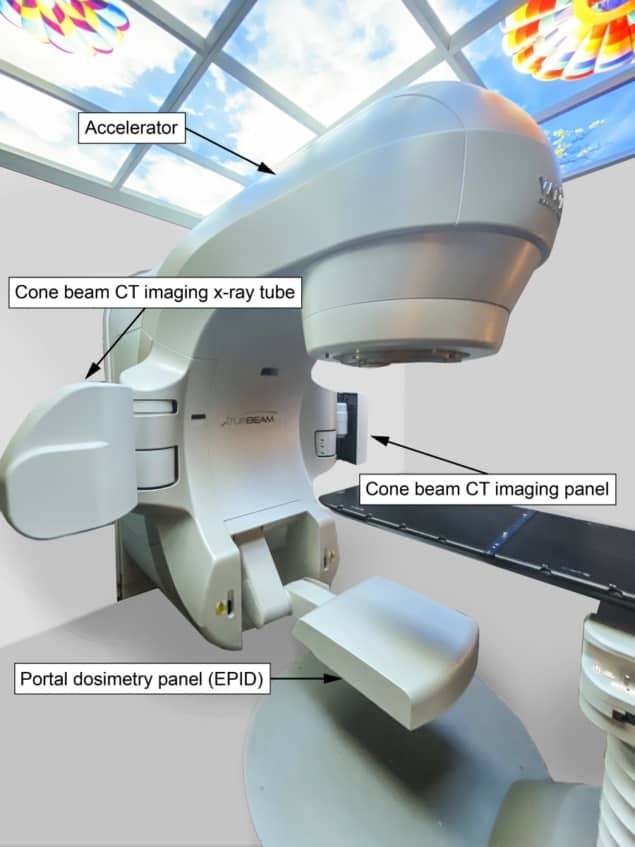
Commercial IVD systems available for EBRT include point detectors placed on the patient’s skin in the treatment field and electronic portal imaging devices (EPIDs), which use the treatment beam to image the patient. The EBRT task group focused on EPIDs, as they are ubiquitous on modern linear accelerators, easy to use, can be automated and can perform 2D or 3D dosimetric verification.
Initially employed for verification of patient set-up on the treatment couch, EPIDs have since been adapted for dosimetric measurements, including IVD. “Developing IVD methods for EBRT requires little investment in hardware, but needs a lot of software and methodology development,” Verhaegen notes.
Within brachytherapy, the main goal of IVD is to catch large deviations from the treatment plan that could affect the clinical outcome. Such deviations arise, for example, from source misplacement, deviations in dwell times or anatomical changes. In particular, the use of real-time IVD could allow treatment interruption and prevent gross errors. IVD systems should also record smaller deviations, enabling inter-fraction adaptation, and provide an estimate of the actual delivered dose.
Currently, there are two IVD methods that could be used with brachytherapy. One involves locating a radiation detector inside the applicator itself. While this approach can identify a variety of errors, it cannot detect movement of the entire applicator with respect to the patient, which would generate serious dose errors. A second option is to put the radiation detector on, or near to, the patient’s skin. This design can detect moving applicators, but the position of the detector itself may be uncertain.
“Both of these methods currently have some uncertainties which should be reduced,” says Tanderup. “Furthermore, treatment verification and error detection relies on a rather complex post-processing of the raw signals from the detectors. As long as software for such post-processing is not commercially available, the IVD methods will not have significant clinical value.”
Several groups, including those of Verhaegen and Tanderup, are currently working to develop novel IVD systems for brachytherapy.
Optimizing IVD
To fully exploit IVD and encourage its clinical introduction, the task group created a wish list for vendors to address. For starters, IVD methods require high sensitivity and specificity, to accurately identify clinically relevant errors while minimizing false alarms. The workflow should be easy to implement in the clinic, but able to trigger alerts when needed. In addition, IVD systems should be fully integrated with treatment planning software and treatment delivery equipment.
Automation could also accelerate the uptake of IVD, which currently involves performing a large number of manual actions, particularly for brachytherapy, and generates large amounts of data, especially with EBRT. “Fully automated systems that interpret discrepancies between planned and monitored therapy are key,” says Verhaegen. “Artificial intelligence could help a lot in catching errors, and even determining their cause and suggesting corrective action. This is a bit of sci-fi for now, but good to aim for – if we can get vendors on board.”
Tanderup and Verhaegen hope that the task group’s recommendations will encourage vendors to get excited by this urgent need for complex treatment verification. “It may be the small start-up companies that rise to the occasion,” Verhaegen tells Physics World. “Hopefully, the recommendations will also motivate clinics with current in-house-developed IVD systems to start collecting clinical data that demonstrate that IVD has clinical value,” adds Tanderup.