
Laser-accelerated proton (LAP) beams may offer a delivery mechanism for proton therapy that has a smaller physical and financial footprint than cyclotron accelerators. LAP irradiation, which is produced when a high-intensity laser beam hits a thin metallic foil, may also cut down proton therapy treatment times from minutes to nanoseconds.
With this potential in mind, researchers are hard at work to confirm how – and if – LAP irradiation differs from conventional proton therapy in other ways.
When the spatial separation between individual proton tracks is small enough, reactive chemical species from individual proton tracks can migrate across tracks. There, chemical species may interact and recombine. For example, hydroxyl radicals commonly associated with indirect DNA damage could recombine into hydrogen peroxide, affecting their availability for reactions with tumour DNA.
In clinical proton therapy, the temporal separation between protons is large enough that physical and chemical interactions between individual proton tracks aren’t possible. Studies on the FLASH effect, however, which occurs on much shorter (0.1–1 s) timescales, suggest that there could be some inter-track interactions in FLASH radiotherapy. Inter-track interactions are therefore also possible with LAP irradiation, which might occur on even shorter timescales than the FLASH effect.
“We need to be sure these different delivery mechanisms do not change the quality of the treatment, so we wanted to investigate the possibility of interactions between protons in such short pulses,” says Shannon Thompson, a final year PhD student at the Patrick G Johnston Centre for Cancer Research at Queen’s University Belfast.
In a study appearing in Physics in Medicine & Biology, Thompson and her collaborators modelled nanosecond laser pulse deliveries and examined inter-track interactions between protons.
Monte Carlo simulations
Thompson used Monte Carlo simulations to explore the physical and chemical interactions that happen immediately after LAP irradiation. She followed chemical species over time for a range of proton energies (0.5 to 100 MeV) and doses and identified the circumstances required for inter-track interactions to occur with LAP.
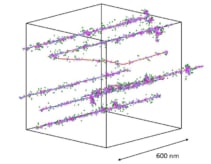
She observed negligible track overlap for all biologically relevant timepoints and proton energies when a clinical 2 Gy radiation dose was delivered. The probability of inter-track interactions increased with time after irradiation, proton energy and dose, with a minimum dose of 23 Gy required before significant track overlap occurred. Thompson also observed no changes in radical yields up to 8 Gy for independently and instantaneously delivered tracks.
“Our results indicate that despite the protons being present at the same time in laser-accelerated delivery, in terms of distance they are too far apart to interact with each other, either directly or indirectly through reactive chemical products,” says Thompson. “This means the initial physical and chemical interactions occurring a few nanoseconds after irradiation would be the same for both laser-accelerated and cyclotron proton therapy delivery. These results suggest there would be no initial differences in DNA damage, a key player in overall cell survival in radiotherapy.”
Results largely agreed with other early experiments on inter-track interactions, which suggest no difference between conventional proton and LAP delivery.
Open questions
Additional research is needed. Cyclotrons accelerate protons for clinical proton therapy up to 250 MeV, while Thompson and her collaborators simulated LAP deliveries up to 100 MeV. Proton beams at higher energies are more likely to experience inter-track interactions, especially with the short temporal distances between pulses in potential LAP deliveries. The simulated water environment also may not mimic inter-track interactions in cells.

Intense radiation pressure enables selective acceleration of carbon ion beams
Thompson says that other research teams are working to simulate LAP beams at higher energies. Researchers are also simulating more detailed chemical reactions in a cell alongside reactions with oxygen.
“There is a long way to go before laser-accelerated proton therapy could be applied within the clinic,” says Thompson. “But it requires a strong inter-disciplinary approach working with physicists, engineers, biologists and clinicians. Such collaborative efforts will enable the greatest advancement in this field to obtain the earliest patient benefit.”
