Why do some thin films of single-wall carbon nanotubes take on colourful hues even though as-synthesized films are usually black? A team of researchers in Finland, the US and China has now come up with a possible answer in a development that could prove useful for future display screens and solar cells.
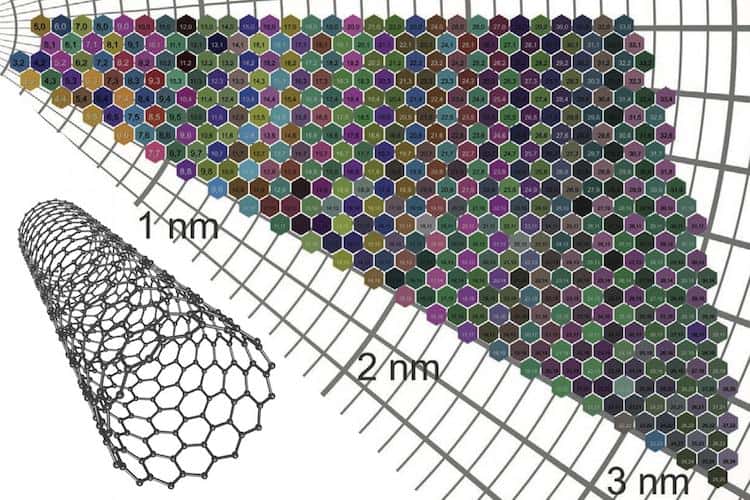
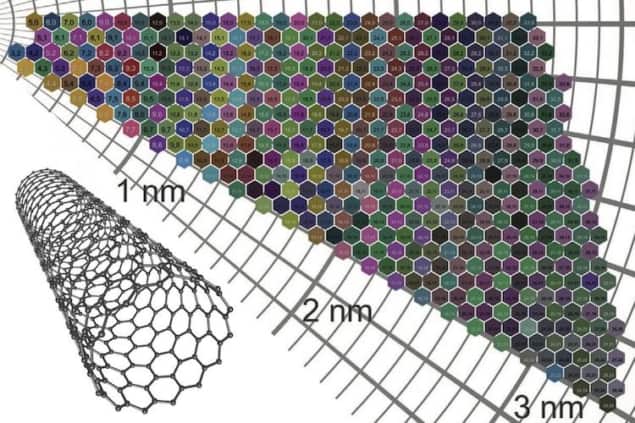
Single-wall carbon nanotubes (SWCNTs) are rolled-up sheets of carbon just one atom thick, with a diameter of about 1 nm. Atoms in these sheets are arranged in a hexagonal lattice and the direction in which the sheet is rolled – its chirality – dictates whether the tube is a metal or a semiconductor. When these SWCNTs are sorted by their diameter or chirality – two traits denoted by an “(n,m)” numbering system – and suspended in a solvent, the resulting solutions are strikingly different in colour. Indeed, each (n,m) type of nanotube has a characteristic colour. Until now, however, the mechanism responsible for this colouration was not fully understood and no theoretical model could successfully predict the colour of a given SWCNT film. Even predicting the range of likely SWCNT colours had proved impossible.
A team led by Esko I. Kauppinen of Aalto University in Finland recently took a step towards understanding nanotube colour by directly fabricating thin films of SWCNTs that were green, brown or silver-grey. The researchers synthesized these nanotubes from carbon monoxide gas using iron nanoparticle catalysts in a reactor heated to 850°C. They then deposited the tubes directly onto a substrate to form the thin films, which did not require any post-solution processing. They made nanotubes with different colours and (n,m) labels by adding carbon dioxide to the reactor.
Quantitative relationship
In their latest work, members of the Aalto team studied dry nanotube films of various (n,m) distributions and analysed the quantitative relationship between the films’ colours and their optical absorption spectra. They then developed a mathematical model based on this relationship that can describe and even predict the colour of films made up of nanotubes with different (n,m) labels.
The Aalto team calibrated and verified its model using a (6,5) nanotube film made by Junichiro Kono and colleagues at Rice University in the US via a technique known as solution (n,m) separation. Together with co-workers at Peking University, China, Kauppinen and colleagues evaluated the light absorption characteristics of the Rice film and its colour. Their analyses revealed that the colour of the film was indeed similar to the colour predicted by their model. “This result proves that the atomic structure – that is, the (n,m) of the tubes in the thin film – and the colour of the nanotube it contains affects how the film absorbs light,” Kauppinen tells Physics World.

Graphene additives promote ‘eco-friendly’ corrosion protection
By then combining the data from different tubes, the researchers were able to build up an “atlas” of 466 different colours of nanotube films. The resulting chart shows that films with diameters of less than around 2.3 nm are more strongly coloured than those with larger diameters.
The work, which is detailed in Advanced Materials, shows that SWCNTs can exist in a range of colours across the visible spectrum. This means they could come in useful for electrochromic devices – for example in displays – and in solar cells, especially as they are electrically conductive, pliable and ductile too. Kauppinen explains that the colour of a screen could be modified with the help of a tactile sensor that could be placed in mobile phones, other touch screens or on top of window glass. They might also be used to make new kinds of environmentally friendly permanent pure-carbon dyes. In the immediate future, however, the researchers plan to use their thin films to manufacture flexible, coloured thin-film field effect transistors with high carrier mobility and fast operation times.