The first direct, real-time observations of an ultrastable glass as it “relaxes” into a supercooled liquid have enabled researchers to quantify a previously mysterious process known as the glass transition. This transition plays a crucial role in numerous fields, including biomedical cryopreservation, drug synthesis, electronic device manufacture and tissue engineering to cite but a few examples. The work could also have implications for solar cells, which often have a coating of patterned glass.
Despite the ubiquitous nature of glass in modern technology and our everyday lives, we do not fully understand it. Though glasses appear solid, their structure is highly disordered, so they are sometimes regarded as liquids with extremely high viscosity. Other mysteries concern how liquids cool and transform into glasses, and vice versa when a glass is heated until it becomes molten. Is this glass transition a distinct thermodynamic state? Or is glass simply a liquid that has been supercooled – that is, one that retains its liquid properties despite being cooled below its freezing temperature?
Similar to crystalline solids
To address these and other unanswered questions, researchers from the Universitat Autònoma de Barcelona (UAB), the Catalan Institute of Nanoscience and Nanotechnology (ICN2), the Polytechnic University of Catalonia (UPC) and the Instituto de Microelectrónica de Barcelona (IMB-CNM) developed a microscopy technique to directly observe what occurs when an ultrastable organic glass is heated to above its glass transition temperature. This “relaxation” process transforms it into a liquid.
The researchers chose to use an organic glass with the chemical name N,N′-bis(3-methylphenyl)-N,N′-diphenylbenzidinem because it transitions to a supercooled liquid state in a way that is similar to that of crystalline solids. In this type of transition, tiny areas of liquid phase form and then gradually grow bigger. This contrasts with conventional glasses, which transition to the liquid state throughout the volume of their structure without any clear divisions between different regions.
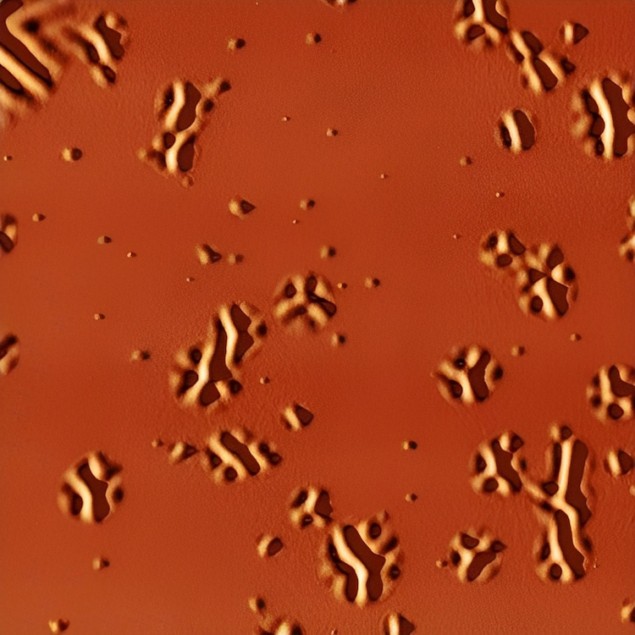
Liquid formation occurs on local, nanoscale regions
The Barcelona team had previously observed this process indirectly using nanocalorimetry, a technique that makes it possible to measure heat capacity in thin films of material. In the new work, which is detailed in Nature Physics, the researchers present a way of observing it directly.
To do this, they sandwiched the organic glass between two layers of a more rigid glass with a higher melting temperature. When they heated the organic glass layer to above its glass transition temperature, the mechanical stress in the softened supercooled liquid regions caused the outer glass layers to deform thanks to the difference in the thermal expansion coefficients between the glassy layers and the silicon substrate onto which the glass is formed. This deformation, which shows up as nanosized bumps, wrinkles and ridges that gradually become bigger, can be observed with an atomic force microscope (AFM).
“Since the formation of the liquid occurs on local, nanoscale regions, the surface corrugation we observed is also local and directly connected to the supercooled liquid beneath,” study team leaders Javier Rodríguez-Viejo and Cristian Rodriguez-Tinoco explain. “The technique allows us to build spatio-temporal maps of the transformation of a thin film glass into its supercooled liquid by directly measuring the distances between the liquid domains that appear. This process can be followed in real time.”

A novel window into ‘smart’ glass
Rodriguez-Viejo adds that the glass transition they observed proved to be highly heterogenous, with large expanses between the emerging liquid regions. “This means the glass does not transform at once across the whole volume into the supercooled liquid, as we may expect for a conventional, liquid-cooled glass, but transforms over a time period that is a million times slower,” he explains. “Indeed, the process we have observed somehow mimics a nucleation and growth mechanism such as that occurring during the formation of a crystalline phase within a glass or the melting of a polycrystal.”
The team now aims to study the glass transition on smaller length scales and over shorter time periods, something that may require members to develop new AFM procedures and protocols. In the longer term, Rodriguez-Tinoco says that findings from the study could help improve industrial methods of glass patterning, which are used to make optical coatings and to control surface roughening in a way that enhances light output in organic solar cells.
