 Merced nAnomaterials Center for Energy and Sensing, the University of California, Santa Cruz (UCSC), the University of California, Merced, and the Lawrence Livermore National Laboratory. The device’s ability to operate at extremely cold temperatures could also make it a good power source for polar expeditions on Earth.
Merced nAnomaterials Center for Energy and Sensing, the University of California, Santa Cruz (UCSC), the University of California, Merced, and the Lawrence Livermore National Laboratory. The device’s ability to operate at extremely cold temperatures could also make it a good power source for polar expeditions on Earth.
Many spacecraft require heating systems to operate in their inhospitable environment. NASA’s Perseverance Rover, for example, recently began a two-year mission to look for signs of ancient microbial life on Mars, where the average temperature is -62 °C, dropping below -125 °C in the winter. Onboard heaters keep the electrolytes in the rover’s batteries from freezing, but the heaters and the energy sources required to power them add weight to the spacecraft payload.
In-between capacitors and batteries
In the trade-off between charge/discharge speed and energy storage capacity, supercapacitors – or, more accurately, electric double-layer or electrochemical capacitors – fall somewhere between batteries and conventional (dielectric) capacitors. Though less good at storing charge than batteries, supercapacitors are better than conventional capacitors in this respect thanks to their porous electrodes, which have surface areas as large as several square kilometres. The double layer that forms at the electrolyte-electrode interface of such devices when a voltage is applied further increases the amount of charge they can store.
Supercapacitors also have some advantages over batteries. They can charge and discharge in minutes – unlike batteries, which take hours. They also have a much longer lifespan, lasting for millions of cycles rather than thousands. And unlike batteries, which work through chemical reactions, supercapacitors store energy in the form of electrically charged ions that assemble on the surfaces of their electrodes.
Hierarchical channels
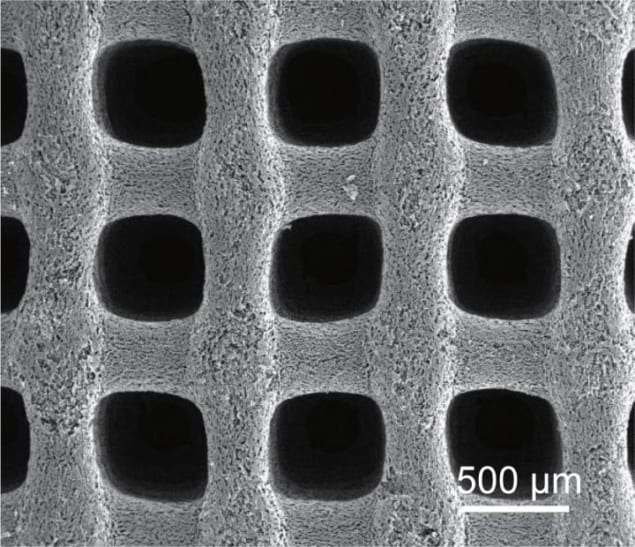
Building on their previous work, the researchers, led by Jennifer Lu of UC Merced and Yat Li of UCSC, used a 3D printing technique called direct ink writing to make their supercapacitor electrodes. They made the ink by combining cellulose nanocrystals (which provide carbon) with a suspension of silica microspheres. The latter serve as a hard template for creating macropores in the lattice structure of the aerogel once it has been freeze-dried.
The pores in the aerogel lattice vary in size from 500 microns to just nanometres, creating a hierarchical structure of channels. These channels significantly increase the rate at which the ions in an electrolyte diffuse through the material, while also minimizing the distance they need to travel.
Advantages over other supercapacitors
The team’s 3D multiscale porous carbon aerogel has a surface area of around 1750 m2/g, and tests show that an electrode made from the material has a capacitance of 148.6 F/g when a voltage of 5 mV/s is applied. The researchers say that this is higher than most other low-temperature supercapacitors. The team also demonstrated that a device containing this electrode allows for ion diffusion and charge transfer at temperatures as low as -70°C. To compare, the lowest working temperatures of commercial lithium-ion batteries and supercapacitors are typically around -20°C to -40°C – values that are limited, as mentioned, by the freezing point of the electrolytes.
Aerogel insulation could provide habitable regions on Mars
The team will now collaborate with scientists at NASA to further characterize the devices’ low-temperature performance. “We will do this by testing them in environments that mimic those of the Moon, Mars and international space stations,” Lu tells Physics World.
The present research is detailed in Nano Letters.
