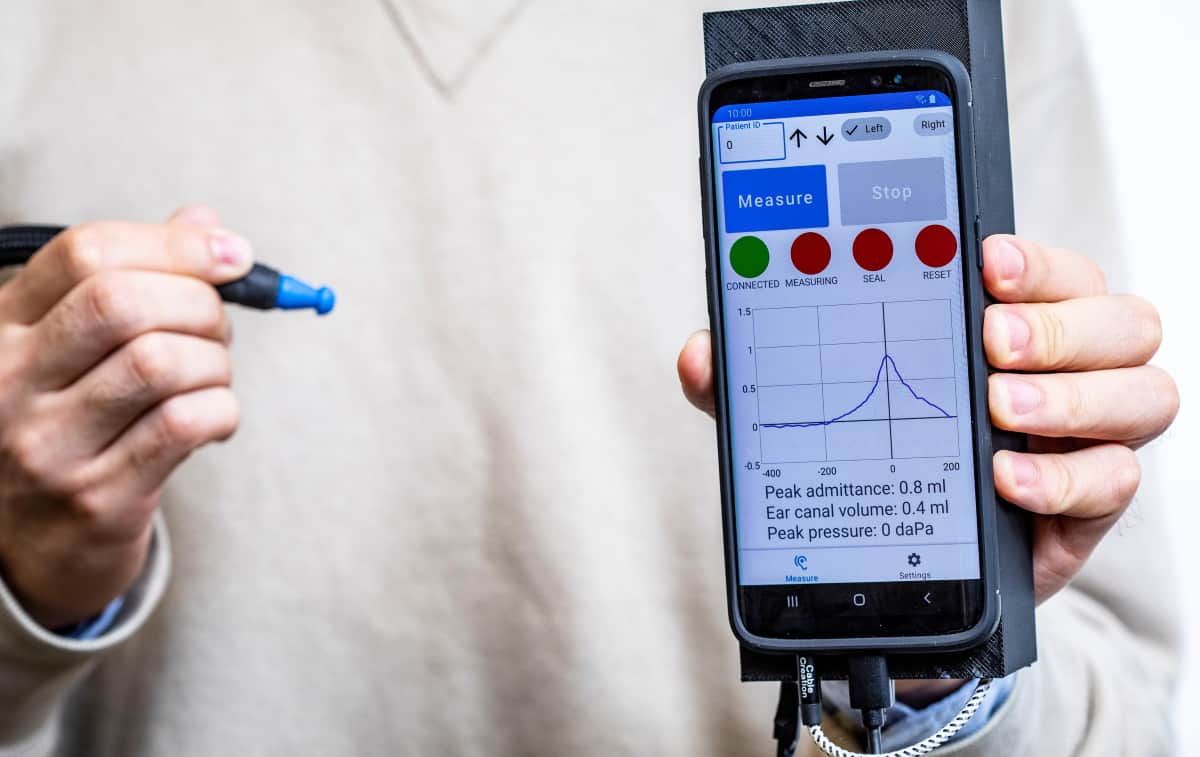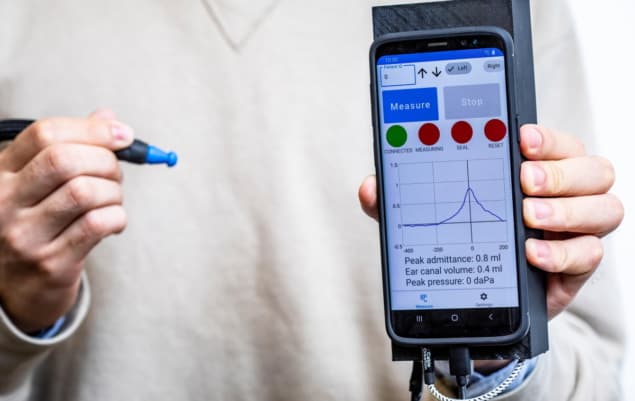
Tympanometry is a test that measures middle ear function by examining the compliance of the eardrum to changing air pressure. The test, used to help diagnose middle ear disorders that could lead to hearing loss, is currently performed using a tympanometer, a device that costs between $2000 and $5000. A team of engineers at the University of Washington in Seattle has now designed a smartphone-based system that performs the same function using off-the-shelf components costing just $28.
The new device comprises a lightweight and portable smartphone attachment that can vary the air pressure of the ear canal and measure eardrum mobility. It automatically detects when a seal has been formed with the ear canal, safely varies air pressure, and generates a tympanogram (a plot of how the eardrum moves) on the smartphone in real time. In an initial clinical study reported in Communications Medicine, 86% of test results agreed with those produced by commercial tympanometers.
The researchers have made their hardware design and smartphone app software (designed to work with the Android operating system) free of charge and accessible to audiologists and developers for use and adaptation. They hope that the device will improve access to tympanometry, particularly in low- and middle-income countries and at geographically remote healthcare facilities.
System design
Led by principal investigator Justin Chan, a PhD candidate at the Paul G. Allen School of Computer Science and Engineering, the team created both handheld and desktop tympanometer systems.

In the handheld version, all of the electronic components fit into a compact 3D-printed enclosure that attaches to the back of a smartphone. An ear probe incorporating pressure and acoustic sensors is connected through 1 m of lightweight air-tight silicone tubes, which provide mobility during measurements. The tip of the probe, which rests securely in a patient’s ear, is a plastic adapter that interfaces with standard tympanometer disposable rubber ear tips.
During a tympanometry test, the air pressure in the ear canal is changed to evaluate eardrum mobility. To achieve this, the system incorporates a pressure transducer made from a stepper motor that precisely moves the plunger of a 5 ml syringe. Moving the plunger by 5.3 mm changes the pressure between -400 and 200 daPa.
A fail-safe device stops the measurement in case of sensor malfunction. Also, if the probe dislodges from the ear canal during a measurement, air pressure returns to ambient pressure.
During the pressure sweep, the system sends a 226 Hz audio tone (the recommended frequency for patients over nine months of age) at 85 dB SPL (sound pressure level) and records the acoustic reflections at a microphone connected to the smartphone. After the measurement, the pressure data are sent to the smartphone using an onboard wireless Bluetooth radio. The synchronized pressure and audio data are then converted into a tympanogram.
Prior to using the system with a smartphone, a one-time sound level calibration is conducted using a sound level meter. The team notes that two individuals unfamiliar with the process were able to perform the entire calibration procedure in less than 5 min after reading instructions.
Clinical study
Tympanometry is helpful for diagnosing middle ear infections, fluid in the middle ear, a perforated tympanic membrane and issues with the Eustachian tube. Children can be especially susceptible to such middle ear problems, and for this reason the designers elected to conduct their initial clinical tests with paediatric patients at Seattle Children’s Hospital.
For the clinical study, two licensed audiologists performed tympanometry on 50 ears from a total of 28 paediatric patients ranging in age from one to 20 years, first with the smartphone device and then with one of two commercial tympanometers (the GSI TympStar Pro or GSI TympStar). Five paediatric audiologists classified the 100 randomized and anonymized tympanograms, with only patient age provided. The agreement between the two device types was on average 86±2% across all five audiologists.

Smartphone-based telemedical eye screening could save sight
“The team is currently researching the utility of the system for infants under nine months,” says senior author Shyam Gollakota. “We are testing the tool with higher frequencies that are used with newborn babies. We are also integrating it with other audiological tests, such as audiometry, to provide a smartphone-based suite for addressing all ear-related conditions.”
Studies are being planned or under way in various low-resource countries. “A new study is currently being set up in Kenya,” Gollakota tells Physics World. “We’ll be announcing details about this in the near future. We are quite excited about all of these. Given the prevalence of inexpensive budget smartphones, particularly in developing countries, our frugal system has the potential to be a screening tool for middle ear disorders in resource-constrained environments.”