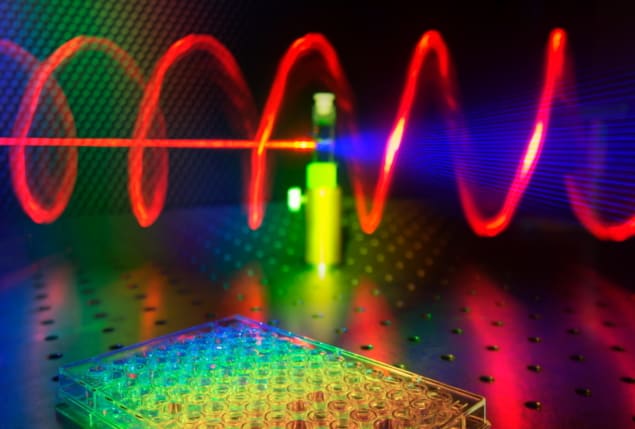
Researchers in the UK and the US have discovered a novel photonic effect that could make it far easier for chemists to assess the chirality of new drug candidates. Led by Ventsislav Valev at the University of Bath, the team achieved the result after synthesizing semiconductor nanohelices that emit intense, twisted blue light along a single direction when illuminated with red light. Their findings could be key to maintaining the rapid pace of modern drug discovery.
Today, drug development techniques are highly automated. Using AI algorithms, robotic chemists can produce millions of mixtures in a single process, creating vast libraries of chemical compounds. To determine whether any of these chemicals may be promising drug candidates, these systems must analyse tiny volumes of them in quick succession. This currently involves the use of microplates containing thousands of wells – each with a volume as small as a cubic millilitre and filled with a unique chemical sample.
Such minuscule sizes create a problem for the latest analytical techniques, especially when assessing the chirality of molecules within a sample – a key measurement for drug analysis. Currently, the optical techniques required to determine chirality require volumes up to 1000 times larger than modern microplate wells. This is a particularly pressing problem, since although some chiral molecules may have identical chemical formulae to their opposite-handed counterparts, their structures can make them ineffective, or even actively harmful to patients when used in drugs.
In the study, described in Nature Photonics, Valev’s team addressed this issue using a novel nanostructure, which mimics the self-assembling behaviours of biological proteins. Firstly, the researchers synthesized twisted ribbons of the semiconductor cadmium telluride – between 5 and 8 µm in length, and roughly 25 nm in width. Without any encouragement, these ribbons braided themselves together to form nanohelices, with chiralities that altered themselves to match those of molecules in their surrounding environments.
Chirality measurements speed up
When the team illuminated the nanostructures with three different wavelengths of red light, they emitted blue light through the process of third-harmonic Mie scattering. This blue light, which twists like a corkscrew around its axis of travel, was emitted 10 times more strongly along the axes of the helices than in sideways directions, making it easy to collect and analyse. This means that when the helices are dispersed evenly throughout a chemical sample, they provide a highly effective way to measure its chirality indirectly.
Valev’s team calculated that this new type of photonic effect could enable chirality measurements within samples 100,000 times smaller than the wells used in current microplate designs. Since red and blue wavelengths are easily distinguished, the technique could enable chemists to rapidly screen the chiralities of vast numbers of compounds, including samples with complexities approaching those of biological tissues.