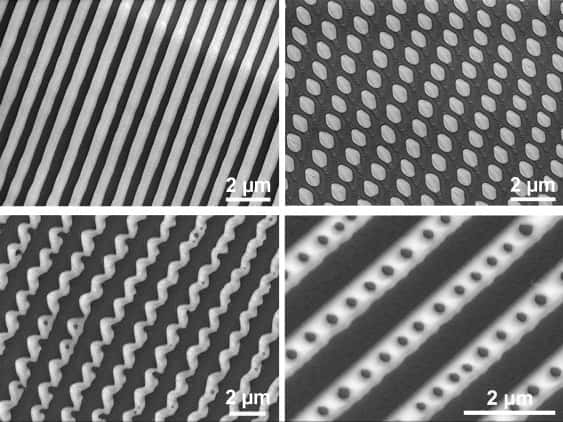
Highly ordered nanopatterns spontaneously form on the surface of alloys as the metal solidifies. This process, which is very different from those that occur in the materials’ bulk, could make it possible to create bespoke patterns on metallic structures and construct devices with applications in electronics and optoelectronics.
Patterns are ubiquitous in nature – think leopard spots and zebra stripes – as well as in human-made systems. Within metallic alloys, micron-sized layers and rod-like structures form when the materials leave the liquid phase and solidify into crystals. This phenomenon mostly occurs via metastable processes known as pattern nucleation and growth. Both processes have long been studied in the bulk of alloys, but researchers had previously paid less attention to their role in forming patterns on the alloys’ surfaces.
Enriched minority phase
Researchers led by Kourosh Kalantar-Zadeh of the University of New South Wales (UNSW) in Sydney, Australia used nanoscale infrared (nanoIR) and surface-enhanced Raman spectroscopy to observe a variety of organized nanopatterns forming on the surface of solidifying gallium-based alloys that contained a small fraction of bismuth (Bi0.0022Ga0.9978, EBiGa). The patterns were dominated by the minority bismuth phase and included alternating stripes, curved fibres, dot arrays and even stripe-dot hybrids.
The researchers observed the front of the bismuth-enriched solid propagating along the material’s surface, generating phase-separated patterns in its wake. The nucleation of this bismuth phase then triggers further growth of the phase along the surface – a surface-catalysed process quite different from conventional bulk solidification.
To understand more about these processes, Kalantar-Zadeh and colleagues turned to collaborators at the MacDiarmid Institute for Advanced Materials and Nanotechnology in New Zealand and RMIT in Australia. These collaborators used molecular dynamics simulations to better understand the UNSW group’s experimental findings. Their calculations revealed that the bismuth atoms appear to move around randomly in a sea (or solvent) of gallium atoms that accumulate at the alloy’s surface – something that classical metallurgy fails to predict.
“An exciting finding”
“This previously ignored surface solidification phenomenon improves our fundamental understanding of liquid metal alloys and the phase transition processes they undergo,” Kalantar-Zadeh explains. “The autonomous surface process we observed could be used as a patterning tool for designing metallic structures and creating devices for advanced applications in future electronics and optics.”
Study lead author Jianbo Tang adds that the surface enrichment of minority phases is “an exciting finding” for efforts to develop metallic structures containing rare or expensive materials for surface-based applications. “Given the diversity of metal species and their abundant combinations, the surface solidification effect could lead to energy-efficient nanoengineering of the surface structures of high-melting-point metals (as has been shown with silver and gold) by incorporating them into room-temperature or low-melting-point metal solvents (such as gallium, indium, tin, bismuth and their alloys),” the researchers write in Nature Nanotechnology.

New material could be used to make a liquid metal robot
The researchers also point out that the minority atoms can cover a large surface. This might be an advantage for applications that need such atoms to be accessible on the surface of fabricated structures, but it could also be a disadvantage if high-purity alloys are required. The researchers therefore recommend that this pattern-forming ability should be carefully considered in advanced manufacturing processes.
The researchers now plan to conduct further studies of liquid metals, believing that the underlying physics and chemistry of these materials – much of which remains unexplored – could have “fascinating” future applications. “Metals in liquid form can be considered as exotic solvents,” Kalantar-Zadeh tells Physics World. “They can thus be used as reaction media to make industrial-grade materials and to convert carbon dioxide with very little energy. They can also be used as the components of intelligent data processors, sensors and actuators, and for developing advanced soft electronic and optical elements.”